The use of liquid electrolytes makes current batteries prone to dangerous thermal runaway reactions, igniting the battery, as seen recently in the Samsung Note 7 phones. To make batteries safer the liquid electrolyte can be replaced by a solid electrolyte, which is not flammable. However, before solid electrolytes can be used the Li-ion conductivity must …
Category: Solid state
Conventional Li-ion batteries contain liquid electrolyte to facilitate the ion transport between the electrodes. However, liquid electrolytes, usually a lithium salt dissolved in an organic solvent, are poisonous, highly flammable, and have a limited stability window. Solid electrolytes do not have these issues and are therefore considered the electrolyte for next generation batteries. Furthermore, the use of solid electrolytes allows more efficient packaging, and therefore higher energy densities. Recently highly ion conducting solids have been discovered that approach liquid electrolytes in terms of Li-ion conduction. However, phenomena related to the interface between solid electrolytes and the electrode(s) that induce high resistance, such as interfacial reactions and bad mechanical contact, need to be addressed to allow successful application of solid electrolytes. Our research focuses on the discovery and characterization of new sodium and lithium ion conductors, and their stability towards electrode materials.
Apr 22
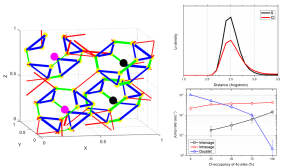
Diffusion in Na3PS4
The wide-spread application of Li-ion batteries could lead to a depletion of world-wide lithium resources. Batteries using Na-ions can replace Li-ions, which is especially interesting for large-scale applications. However, for large-scale the inherent dangers of liquid electrolytes become bigger, thus there is a large interest in solid electrolytes for these applications. We investigated the sodium-ion …