 In an effort to keep up with the increasing demands for energy storage, lithium sulfur (Li-S) batteries receive a lot of research attention as they are considered one of the most promising technologies to replace the current lithium-ion batteries. Sulfur has the ability to reversibly store 2 electrons per atom and is an abundant, benign, and low cost material. However, sulfur also has an extremely low electrical conductivity. Using a highly resistive active material in a battery normally means severe (ohmic) losses, and is therefore seen as a large obstacle for the efficient storage of energy. This is in conflict with the relative ease with which sulfur can be applied as electrode material, evidenced by the many promising results obtained for Li-S batteries over the last few years.
In an effort to keep up with the increasing demands for energy storage, lithium sulfur (Li-S) batteries receive a lot of research attention as they are considered one of the most promising technologies to replace the current lithium-ion batteries. Sulfur has the ability to reversibly store 2 electrons per atom and is an abundant, benign, and low cost material. However, sulfur also has an extremely low electrical conductivity. Using a highly resistive active material in a battery normally means severe (ohmic) losses, and is therefore seen as a large obstacle for the efficient storage of energy. This is in conflict with the relative ease with which sulfur can be applied as electrode material, evidenced by the many promising results obtained for Li-S batteries over the last few years.
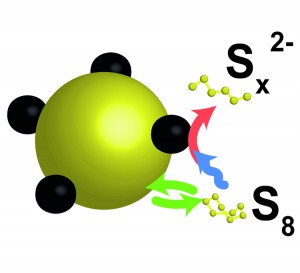
In our recent publication, we assessed the implications of the non-conductive characteristics of sulfur for the operating principles of the Li-S battery. By applying a simple but effective battery cell we showed that the dissolution of elemental sulfur, despite its low solubility in the organic solvent of the electrolyte, plays a significant role in the operation mechanism of the Li-S battery. From the results it was evident that sulfur can participate in the redox reactions as a dissolved molecule. In doing so the low conductivity of bulk sulfur is effectively circumvented; the electrons do not need to go through the bulk sulfur, but the dissolved sulfur goes to the electrons. Based on the electrochemical response of the special cells the researchers furthermore indicate that the non-conducting solid sulfur can be utilized indirectly by interactions with intermediate products that are formed during battery operation. These factors explain the relative ease with which this insulator can be applied as electrode material in liquid electrolyte cells.
