 The use of liquid electrolytes makes current batteries prone to dangerous thermal runaway reactions, igniting the battery, as seen recently in the Samsung Note 7 phones. To make batteries safer the liquid electrolyte can be replaced by a solid electrolyte, which is not flammable. However, before solid electrolytes can be used the Li-ion conductivity must be equally good as in liquid electrolytes.
The use of liquid electrolytes makes current batteries prone to dangerous thermal runaway reactions, igniting the battery, as seen recently in the Samsung Note 7 phones. To make batteries safer the liquid electrolyte can be replaced by a solid electrolyte, which is not flammable. However, before solid electrolytes can be used the Li-ion conductivity must be equally good as in liquid electrolytes.
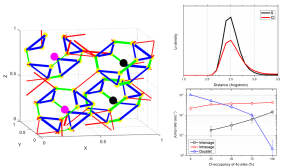
Currently several solid electrolytes are known with Li-ion conductivities comparable to that in liquid electrolytes, one of which are lithium argyrodites. To gain a better understanding of Li-diffusion in lithium argyrodites density functional theory molecular dynamics simulations have been performed.The simulations showed that the position of halogen atoms in the crystals has a large impact on the Li-ion conductivity, and that altering the halogen positions in lithium argyrodites during synthesis could increase the Li-ion conductivity of these materials.
The simulation results show that the Li-ion conductivity in these materials can a factor of 2 larger than the currently highest value. (Article)
